|
 |
a) |
 |
b) |
 |
c) |
 |
d) |
 |
e) |
Solid phase diffusion bonding
Bonding in the solid phase is mainly carried out in vacuum or a protective atmosphere, with heat being applied by radiant, induction, direct or indirect resistance heating. Pressure can be applied uniaxially or isostatically. In the former case, a low pressure (3-10MPa) is used to prevent macrodeformation of the parts (i.e. no more than a few percent). This form of the process therefore requires a good surface finish on the mating surfaces as the contribution to bonding provided by plastic yielding is restricted. Typically surface finishes of better than 0.4µm RA are recommended and in addition the surfaces should be as clean as practical to minimise surface contamination.In hot isostatic pressing, much higher pressures are possible (100-200MPa) and therefore surface finishes are not so critical, finishes of 0.8µm RA and greater can be used. A further advantage of this process is that the use of uniform gas pressurisation allows complex geometries to be bonded, as against the generally simple butt or lap joints possible with uniaxial pressurisation.
Where dissimilar materials need to be joined in the solid phase (and in particular metal to ceramic joints), it is possible to introduce single or multiple interlayers of other materials to aid the bonding process and to modify post-bond stress distribution.
Liquid phase diffusion bonding/diffusion brazing
This technique is applicable only to dissimilar material combinations or to 'like' materials where a dissimilar metal insert is used. Solid state diffusional processes lead to a change of composition at the bond interface and the bonding temperature is selected as the temperature at which this phase melts.Alternatively, with the dissimilar metal insert, it melts at a lower temperature than the parent material. Thus a thin layer of liquid spreads along the interface to form a joint at a lower temperature than the melting point of either of the parent materials. A reduction in bonding temperature leads to solidification of the melt, and this phase can subsequently be diffused away into the parent materials by holding at temperature, Fig.2.
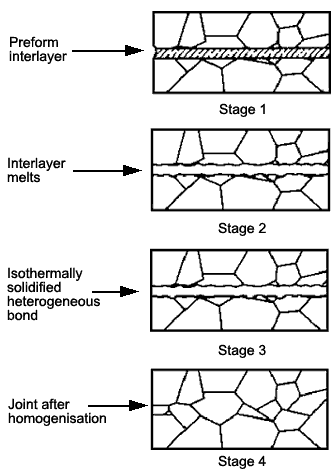 |
Fig.2 Schematic illustration of the steps involved in making a diffusion-brazed joint |
The technique has been used particularly for the bonding of aluminium alloys where eutectics can be formed with copper, silver or zinc to assist in the break-up of the stable aluminium surface oxide.
Superplastic forming/diffusion bonding
This technique has been developed specifically within the aerospace industry, and its industrial importance is such that it should be considered separately here. The process is used commercially for titanium and its alloys, this material being one that exhibits superplastic properties at elevated temperatures within defined strain rate conditions. These conditions of temperature and pressure coincide with the conditions required for bonding, and therefore the two processes have been combined into one manufacturing operation either in sequence or together. The process (known as SPF/DB or more correctly DB/SPF) is used to produce stiff sandwich structures for airframe parts, or the wide chord, hollow fan blades for aeroengines. Both these involve skins with internally bonded structures as reinforcing elements.Summary
Variants of the diffusion bonding technique are available, offering scope for the joining of many new materials and configurations. Because of the capital equipment costs, surface preparation requirements and long bonding times, the process is restricted to high value, relatively low production components. However, its versatility for unusual material combinations and its relative ease of use for titanium alloys (including intermetallics) will ensure the process continues to be developed for specific application requirements.| ◄back | |
| ▼home |
Diffusion bonding of titanium alloys
Microstructure from an electron beam diffusion bond in Ti-48Al-2Mn-2Nb alloy |
 |
Diffusion bonding of titanium alloys is an easy but comparatively slow joining technique. Work at TWI has shown that bonding using an electron beam to heat locally just the interface area considerably speeds up the process.
Usually a whole component and its jigging must be heated to temperature. However, a large thermal mass takes quite some time to cool down. With local heating, both the time at temperature and the cooling period are reduced, allowing a faster turn around. The flexibility of electron beam processing will also allow several joints to be welded at once, and gives a reduced energy consumption.
The process has been demonstrated by TWI for Ti6Al-4V and for several ![]() -TiAl intermetallic alloys, including Ti-48Al-2Cr-2Nb and Ti-48Al2Mn-2Nb. In addition
it can be used to join dissimilar titanium alloys. Microstructures across the welded area are essentially the same as those formed by conventional diffusion bonding.
-TiAl intermetallic alloys, including Ti-48Al-2Cr-2Nb and Ti-48Al2Mn-2Nb. In addition
it can be used to join dissimilar titanium alloys. Microstructures across the welded area are essentially the same as those formed by conventional diffusion bonding.
Another advantage of using this process is that it operates at temperatures below those at which phase transformations occur.
| ◄back | |
| ▼home |
Diffusion bonding - Ceramics and ceramic/metal joints
Diffusion bonding is a solid phase process achieved via atomic migration with no macrodeformation of the components. Initial surface flatness and cleanliness are essential. Surface roughness values of less than 0.4microns are required and the samples must be cleaned in acetone prior to bonding. Typically the process variables range from several hours at moderate temperatures (0.6Tm ) to minutes at higher temperatures (0.8Tm ), with applied pressure. The process is most commonly used for titanium in the aerospace industry. However, ceramics can be diffusion bonded to themselves and to metals.Ceramic-ceramic diffusion bonding is difficult to achieve unless either diffusion aids or (more commonly) secondary phases are present. These are most typically glassy phases at grain boundaries.
Bonding of thin shims to build up complex 3D structures is one of the most up-to-date applications for this technology. This has been achieved for rapid prototyping of alumina components. Some may argue that this is sintering; and in reality there is little difference in the mechanics of diffusion bonding and sintering.
Diffusion bonding usually takes place in a uniaxial press (hot isostatic pressing can be used but requires more complex fixturing) heated via elements or induction units. This also presents a restriction on the size of components that be processed. However, a more recent innovation uses microwave heating and this has been shown to produce excellent bonds in a matter of minutes, but still for components no bigger than a shoebox.
It is also possible to produce ceramic-metal diffusion bonds; and, as for ceramic-ceramic diffusion bonding, a combination of time, temperature and pressure are required. Figure 1 shows a schematic of the bonding process, note that the metal deforms at the macro level to the ceramic. Even so, good flat mating surfaces are beneficial.
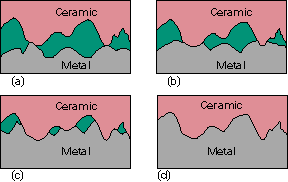 |
Fig.1. Sequence for diffusion bonding of ceramics to metalsa) Hard ceramic and comparatively soft metal surfaces come into contact |
There is usually an added complication when joining dissimilar materials - the differences in coefficient of thermal expansion (CTE). This can cause strains to develop at the interface which can cause premature failure of the bond. This can be overcome by ensuring correct design of the bond line and/or by using interlayers, which have CTE's intermediate to those of the materials to be joined and often have a low elastic modulus. Appropriate stacking of interlayers (ranging in thickness from microns to mm's) can allow diffusion bonding to take place.
In the electronics industry, alumina and aluminium nitride have been bonded to copper using a thin gold interlayer. Gold is an exceptionally good interlayer and diffusion bonds can be produced at temperatures as low as 300°C.
Diffusion - or more accurately ionic migration - forms the basis of electrostatic bonding. Electrostatic bonding requires that the surfaces being joined must be as flat and clean as possible. The components to be bonded are heated in a vacuum and pressure is applied. Typical conditions would be 3MNm-2 and 400°C. When the required temperature has been achieved a DC voltage of about 100V is applied and the metallic component is held positive. The nonmetallic component must contain mobile ions such as Na+. The process has been successfully applied to glass and ceramics such as beta-alumina. Figure 2 shows an electrostatically bonded silicon-glass transducer.
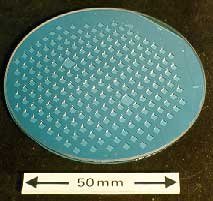 |
Fig.2. Electrostatically bonded silicon glass transducer |
Electrostatic bonding offers several advantages for joining in the electronics and optics industries. These include bonding without interlayers, which is a source of contamination, and relatively low bonding temperatures.
A further variation of the conventional diffusion bonding process involves the formation of a transient liquid phase. The best example of this is the bonding of copper to alumina. The process, known as direct copper bonding, involves copper in contact with the alumina in a slightly oxidising atmosphere at a temperature of 1070°C. Under these conditions the surface of the copper oxidises and forms an eutectic liquid. The liquid reacts with the alumina and on solidification a copper aluminate phase is formed at the interface, producing a bond between the copper and alumina.
| ◄back | |
| ▼home |